How can I explain with illustrations the cohesive forces of water molecules?
1 Answer
When I teach about the intermolecular forces in water I get the students to bet on how many water droplets they can place on top of a penny without any falling off. They usually bet about 10, and then find out when they do the experiment that with care about 40 drops can be added.
We can then go on to talk about how hydrogen bonds form between the lone pairs on the oxygen atoms in water molecules and the
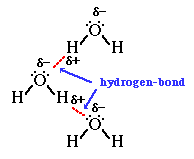
Finally we'd look at other anomalous properties of water such as relatively high melting and boiling point (for a Group 6 hydride), and the fact that ice floats as it has a lower density than liquid water.

