#sf(""^24Na)# undergoes #beta# decay. A 208g sample decays such that 13g remain. What is the 1/2 life ?
2 Answers
If we begin with 208 grams at the end of 1 half-life there would be 104 grams remaining, at the end of the 2nd half-life there would be 52 grams, at the end of the 3rd half-life there would be 26 grams and at the end of the 4th half-life there would be 13 grams.
The total time of decay is 60 hours and this represents 4 half-lifes.
#(60 hours)/(4 half-lifes) = 15 hours/half-life
The half-life for Sodium-24 is 15 hours.
The above answer is a good answer for that specific question so I will give an answer for a more general situation where the numbers may not work out so nicely.
For a random event such as the decay of a radioactive nucleus the rate of decay depends only on the number of undecayed atoms.
We replace the proportional sign by a constant , in this case the Greek letter lambda
Since the number of atoms is decreasing we can write:
You may have seen the same thing if you have studied 1st order reaction kinetics - the theory is just the same and applies to many natural processes referred to as "exponential decay".
A typical decay curve looks like this:
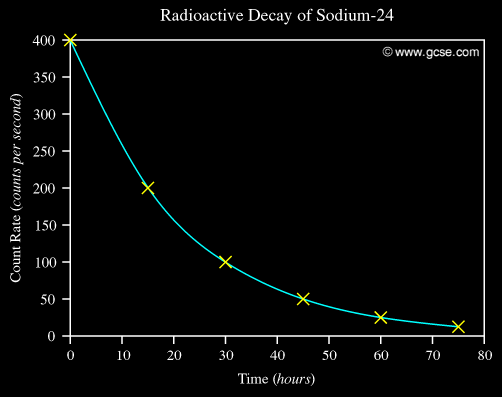
By doing some mathematics using integration (which I won't go into here) we get:
To turn this expression into a more usable form we take natural logs of both sides to give:
So
We can use mass in grams for numbers of atoms (since they are proportional) so we can put in the numbers to get
The faster the decay (related to
So:
So you can use a particular value of


