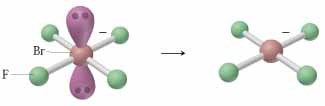
What is the molecular geometry of BrF4- ? A) square planar B) square pyramidal C) seesaw D) tetrahedral
1 Answer
A) square planar.
Explanation:
The answer is A) square planar.
Start from the Lewis structure of the tetrafluoroborate ion,
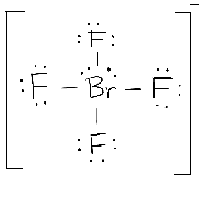
The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of 8 of the 36 valence electrons. The fluorine atoms will each have 3 lone pairs attached, bringing the number of used valence electrons to 32.
The remaining 4 valence electrons will be placed on bromine as lone pairs.
The bromine atom is surrounded by 6 regions of electron density - four single bonds and 2 lone pairs, which means that its steric number will be equal to 6.
According to VSEPR Theory, the molecular geometry will be square planar -