How is the peak affected in #C^13 NMR# by the number of H's attached to the carbon?
1 Answer
In a "normal"
That's because most
The couplings are removed by applying a continuous second radio signal that excites all the protons and cancels out their couplings with the
Thus, the spectrum of 2-bromobutane consists of four singlets.
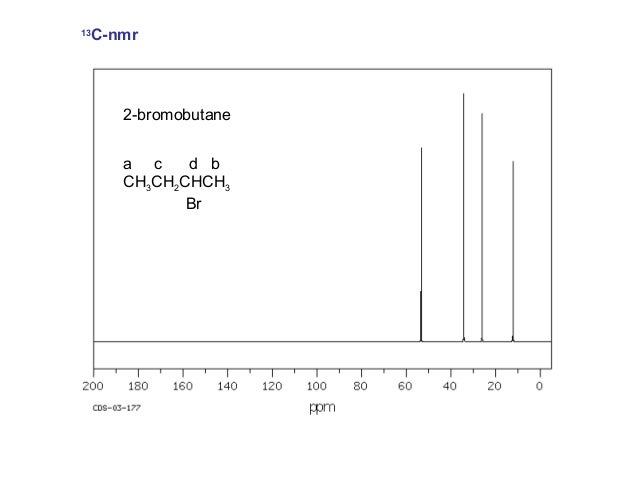
However, there is a technique called off-resonance decoupling , in which only the protons bonded to a given carbon atom split its signal.
Thus, a
So, the off-resonance decoupled spectrum of 2-bromobutane consists of two quartets, a triplet, and a doublet.



