How many sigma and pi bonds are in the following molecules?
#NF_3#
#CS_2#
#ClF_5#
#HC Cl_3#
#XeF_2#
1 Answer
Below.
Explanation:
 )
)
 )
)
 )
)
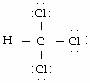
 )
)
Imagine the structures in your head, using knowledge of how many bonds each atom can have. A single bond is a
Edit: images been included

