Question #8edc0
1 Answer
Dec 20, 2016
The final concentration is
Explanation:
To obtain the final molarity, we can use the equation below:
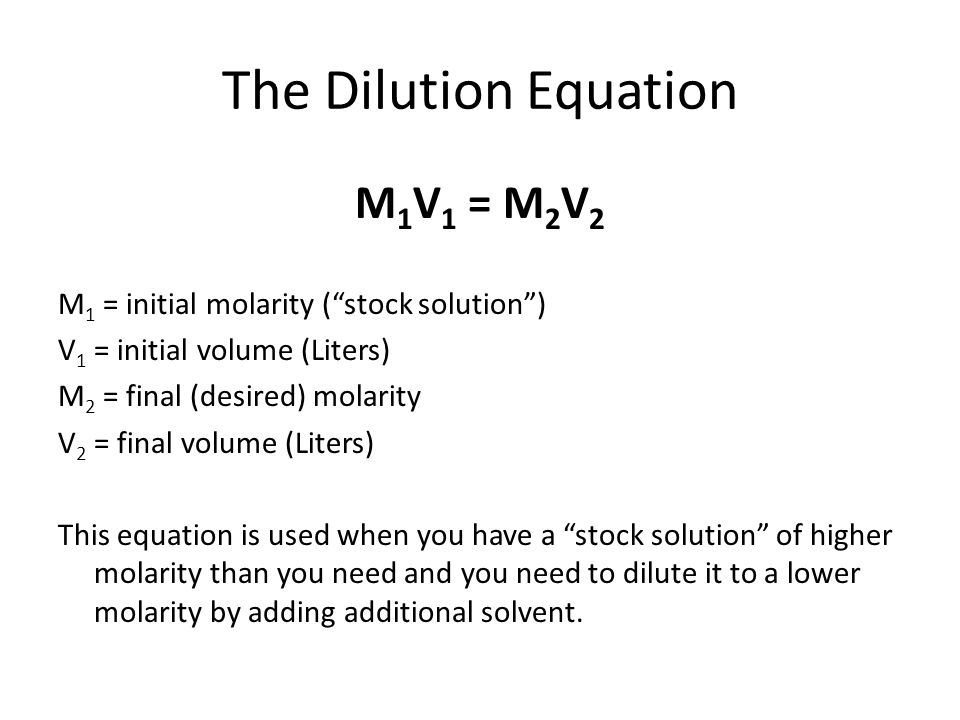
According to the information provided, we know the following information:
Since we don't know
Now, just plug in the known values and solve for the second concentration:

