What is a double-replacement reaction in chemistry?
1 Answer
See explanation
Explanation:
Double replacement (sometimes referred to as double displacement) reactions are when parts of ionic compounds are switched to form two new ionic compounds.
Heres an image:
![enter image source here]
(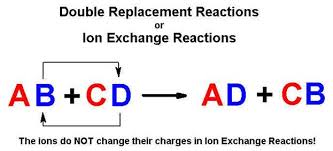 )
)
The way I think of it, since we're dealing with ionic compounds, is that when I write out a reaction I begin like this.
We swap the anions so that
Heres an real example:
Say you have Sodium Cyanide and you react it with Hydrogen Bromide:
Well we have to remember what are the charges of cation and anion. Since this is an easy example, they all have a charge of +1 for the cations and -1 for the anions but we can just write
Next we can just switch the anions to get
(This forms Hydrogen cyanide gas by the way)
I'm not the best at explaining chemistry so if this was confusing I apologize and recommend checking out Tyler DeWitt's video on "Types of Chemical Reactions" as well as the his videos.

