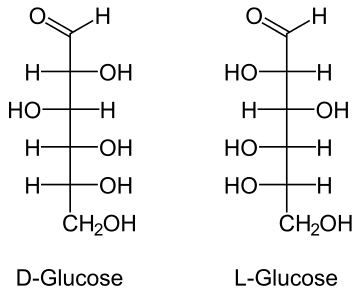
#"D-carbohydrates"# and #"L-carbohydrates"# are visually best seen in their straight chain forms.
Let's take #color(orange)"D-glucose"# and #color(magenta)"L-glucose"#, for example.
 )
)
When numbering, the Carbon that is part of the #color(blue)"aldehyde"# functional group is numbered #color(blue)"C1"# and is numbered so due to aldehydes given highest priority. #"C2"# and on are counted going down the straight chain molecule.
Both of these molecules shown are glucose. The only difference is that they both differ in their absolute configurations at their #color(blue)"chiral Carbon centers"#. Since at each chiral center- the chiral centers being #color(blue)"C2, C3, C4, and C5"# - their absolute configurations are opposite to that of the mirror image, they are considered #color(blue)"enantiomers"# of each other.
#color(blue)"Enantiomers"# are isomers which differ in their absolute configurations at every chiral center. This means if you were to assign #"(R)"# or #"(S)"# at #"C2, C3, C4, and C5"# in the #"D form"# of glucose, it would have either the #"(R)"# or #"(S)"# absolute configuration and its mirror image, the #"L form"#, would have the EXACT OPPOSITE absolute configuration.
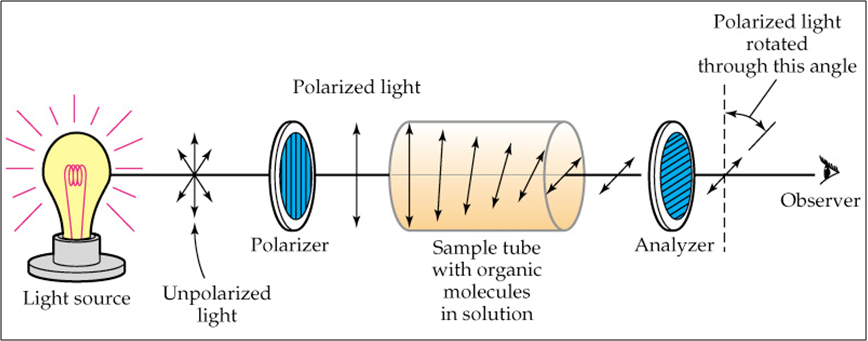
Another property of enantiomers is that they are #color(blue)"optically active"#. This means they can rotate plane polarized light to a certain degree. If one enantiomer rotates plane polarized light at an angle of #color(orange)[+34^(@) ("the + indicates to the right or dextrorotary")#, its enantiomer would rotate the plane polarized light at an angle of #color(magenta)[-34^(@) ("the - indicates to the left or levorotarty")#. They would have the same magnitude but rotate in different directions.
 )
)
Note: The D and L in the carbohydrates just refers to the direction the hydroxyl group of the chiral carbon furthest away from the aldehyde/ketone group faces (D = OH faces to the right side, L= OH faces to the left side). d, l, D, L, R, and S have no relation to each other.
 )
)  )
) 
