Let me first quickly go over a little bit about #color(red)"Carbohydrate chemistry"# and then I will get to the answer.
#color(red)"Lactose"# and #color(red)"sucrose"# are both carbohydrates or more commonly referred to as sugars. More specifically, they are #color(red)"disacharrides"# as they are both made up of 2 sugar units.
#color(blue)["Figure 1. The disacharrides Sucrose and Lactose in their ring forms"#
 )
)
#color(white)#
#color(blue)[---------------------#
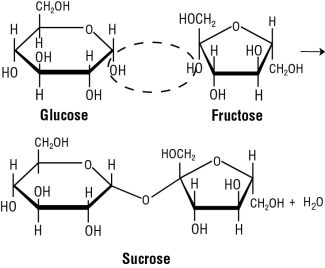
Let's look at sucrose first. Sucrose is made by the condensation reaction of a #color(red)"glucose molecule"# and a #color(red)"fructose molecule"#. What essentially happens is the hemiacetal of the glucose molecule reacts with the hydroxyl group of the fructose molecule. This reaction ends up forming an #color(red)"O-glycosidic bond"#. [#color(blue)["Figure 2"#] (a reaction you should be familiar with from organic chemistry)
#color(blue)["Figure 2: Sucrose formation"#
#color(blue)[---------------------#
#color(white)(a)#
Figure 3 below shows a general hemiacetal/hemiketal and acetal/ketal reaction.
#color(blue)"Figure 3: Hemiacetal/hemiketal and acetal/ketal general reaction"#
 )
)
Looking at the straight chain form of glucose on the left side of the figure, the carbonyl carbon (C1) reacts internally with the hydroxyl group - the one attached to C5 - to form a 5 membered ring (the right side of the figure). The carbon labeled 1 is the anomeric carbon
#color(blue)[---------------------#
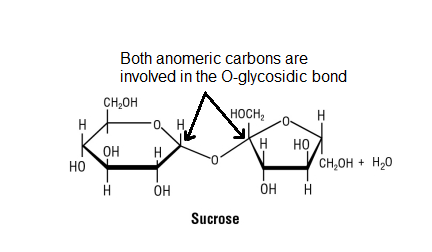
The cool thing about anomeric carbons is that depending on their stereochemistry two #color(red)"anomers"# (isomers) can exist - the #color(red)[alpha]# or the #color(red)[beta]# form. At equilibrium, the 2 anomers and the straight chain form will exist with the #beta# form predominating in solution. These two forms can interconvert spontaneously in a process called #color(red)"mutarotation".#
#color(blue)["Figure 5: Mutarotation where equilibrium exists between cyclic"#
#color(blue)"and straight chain forms of the sugars"#
#color(white)#
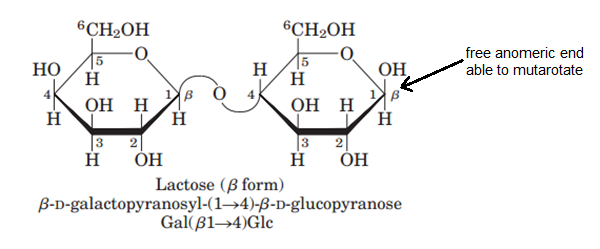
Lactose on the other hand, can mutarotate since it does have an available anomeric carbon.
#color(blue)["Figure 7: Lactose and its free anomeric end"#
 )
) )
) 
