How do transition elements form coloured compounds?
2 Answers
Movement of electrons between shells
Explanation:
I believe that due to the d orbitals being inside the outer s orbital, electrons are able to move into that s orbital if they have the required energy. They then drop back down to the d one emitting a photons at specific frequencies giving them their colour.
Explanation:
Transition metal ions are not coloured on their own.
It is only when they form complexes with other ions or molecules that they become coloured.
In a transition metal, the

However, when the metal ion is complexed with other ions or molecules, some of the
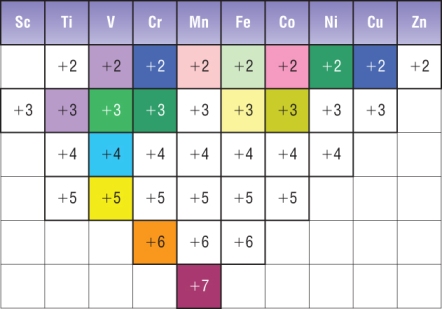
One common pattern is shown in the diagram above.
The difference in
Thus, an electron in a lower
The non-absorbed light is reflected back to our eyes, so we would probably see a blue or green colour.
Note: a transition metal ion that has zero or ten
Here are the colours of some transition metal ions in aqueous solution.