What is the relationship between atomic radius and ionization energy?
1 Answer
Inversely proportional.
Explanation:
-
Ionization energy by definition is the energy required to move an electron from a gaseous atom (or ion).
-
Atomic radius is the measure of the size of an atom. (An estimate of the radius, or distance, between the nucleus and the electron on the furthest occupied shell.
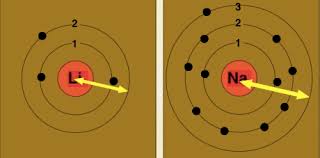
As for the atom structure, the positive nucleus is in the center, and the negatively charged electrons are around, so the only force (relatively) acting on the electron that will affect the ionization energy is the electrostatic force by the nucleus.
Therefore the closer the electron to the nuclear the higher the attraction force, and thus the higher the energy required to overcome this attraction and remove the electron.
Therefore the smaller the radius the higher the ionization energy, and the bigger the radius the lower the energy need.

