Question #4d7d2
1 Answer
Nov 6, 2017
Explanation:
Cationic surfactants are pH-dependent primary, secondary, or tertiary amines: Primary and secondary amines become positively charged at pH < 10.So to make the surfactant work it has to be highly alkaline causing the compounds to react,lose aromacity.
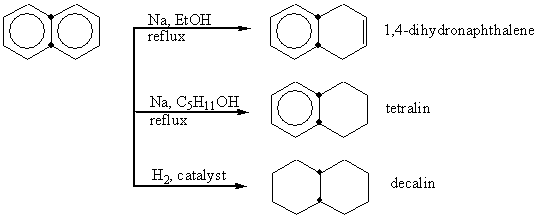
Napthalene makes a large part of the aromatic hydrocarbons in kerosene. Reaction with bases make it lose its aromacity like napthalene's one ring loses its aromacity in this reaction
Even aniline is not an effective cationic surfactant

