Question #f1a87
1 Answer
Dec 1, 2017
Explanation:
This is a double displacement, or precipitate, reaction where the metal cations of two products swap anions:
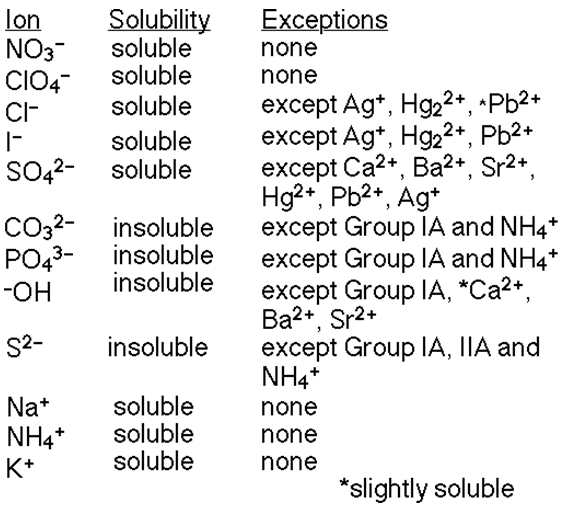
The reason this reaction occurs is because 1 of the products is insoluble in water, meaning when this reaction takes place in water a solid precipitate would form indicating a chemical reaction.
The solubility of each compound is determined by the solubility rules below: