How to arrange the following atoms and ions in order of increasing atomic size?: #"Rb", "Ag", "O"^(-2), "Al", "O", "Cs", "Al"^(+3), "Si"#
I know that atomic size trend decreases left to right and increases top to bottom on the periodic table, so it should be like #"O", "Al", "Ag", "Rb", "Cs"#
But what about the ions #"O"^(-2)# and #"Al"^(+3)# ? Where would they be put in this order?
I know that atomic size trend decreases left to right and increases top to bottom on the periodic table, so it should be like
But what about the ions
2 Answers
The atomic radius of these elements and ions will increase in the following order:
The semi-colons (;) indicate a new period (row). See below for
Explanation:
Atomic size decreases left to right on the periodic table because the attractive force of the protons on the same number of rings of electrons increases for each element as we move left to right. This effectively pulls the electrons closer to the nucleus.
Atomic size increases moving top to bottom because of the addition of another ring of electrons to each element as we move from top to bottom. Outer electrons can move further away from the nucleus.

The oxygen ion you have with
The aluminum ion is much smaller than the elementary aluminum with the loss of three electrons. In this case, we have 13 protons attracting ten electrons. As a result, the
The rest line up according to the periodic table as mentioned above. There is a list of atomic (covalent) radii here:
http://periodictable.com/Properties/A/AtomicRadius.v.html
Note the trends indicated above apply only to Covalent Radii and not to van der Waals Radii.
http://chemguide.co.uk/atoms/properties/atradius.html
Explanation:
Here's how I would do it.
Arranging the atoms
It is fairly easy to arrange the atoms according to atomic size.
We know that atomic size increases from right to left and from top to bottom in the Periodic Table.

The smallest atoms are at the top right and the largest atoms are at the lower left of the Periodic Table.
Thus, we predict the following order:
How to fit the ions
(a) Fitting
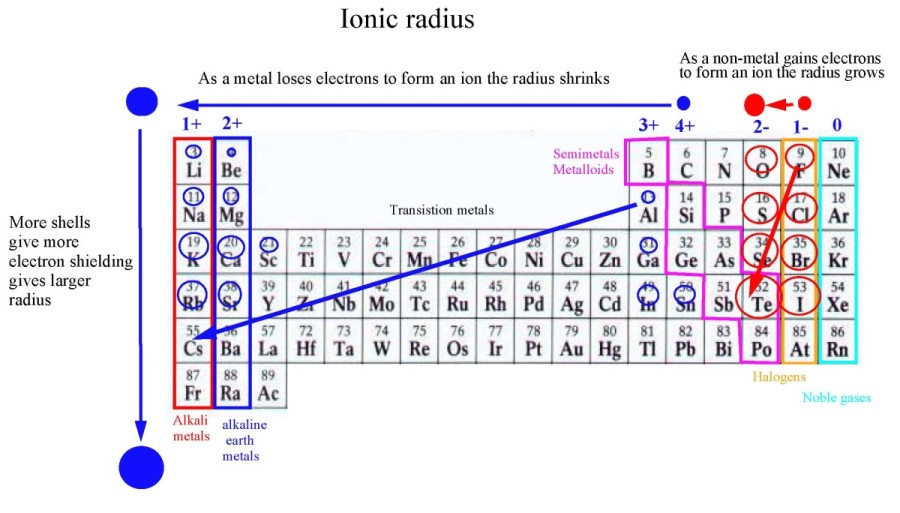
When
I would move it to the other side of
This gives the order
(b) Fitting
This one causes the most uncertainty.
When you add two electrons, the electron repulsion is so great that the ion swells to the size of atoms in the next row of the Periodic Table.
I would put it between
This gives the order

