Question #b8881
1 Answer
They both describe electrons as being in differing energy levels or 'shells'
Explanation:
Both models account for electrons existing in different shells (or 'orbits') relative to their energies.
The key difference between the two models is in the way the electrons are described as orbiting an atoms nucleus.
In the Bohr model of an atom, electrons are considered more as 'satellites' orbiting an atoms nucleus. They are described as particles.
 )
)
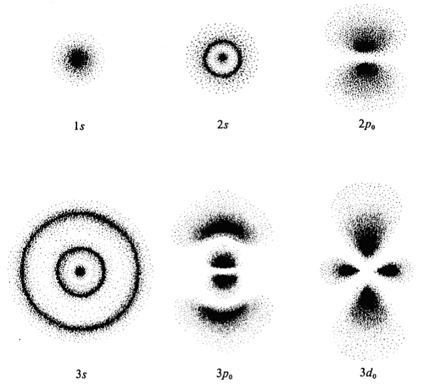
In the quantum model, however, electrons are described as having a likely probability of being found in a given space around the atoms nucleus. These 'spaces' are given as orbitals or 'shell's but are not like a neat circular orbit around a central point. They are regions of space that are likely to have electrons within them. Electrons are said to behave like waves as well as particles. This gives rise to 'wave particle duality' - an underpinning feature of quantum mechanics.