What are some nonpolar covalent bond examples in living things?
1 Answer
Some examples are:
Explanation:
Covalent bonds are common in the molecules of living organisms. This bonds are created is by sharing electrons. The more electrons they share the stronger the bond will be.
Non-polar covalent bonds appear between two atoms of the same element or between different elements that equally share electrons.
-
#O_2# and#CO_2#
#O_2# is non-polar because the electrons are equally shared between the two oxygen atoms. The same case is with#CO_2# .
![https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/110/2016/05/02211833/Figure_02_01_11jpg]()
Breathe in and out! When you inhale,#O_2# comes in your lungs, and when you exhale,#CO_2# goes out. This process happens because#CO_2# and#O_2# are nonpolar molecules.

-
#CH_3#
Our intestinal gas/ flatus is produced as a byproduct of bacterial fermentation in our colon.

Click here for: Why is#CH_4# a non-polar molecule?
![https://s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/110/2016/05/02211833/Figure_02_01_11jpg]()
-
DNA
Each strand of the DNA is polar, but each strand with its polarity neutralizes the molecule of the DNA in total.

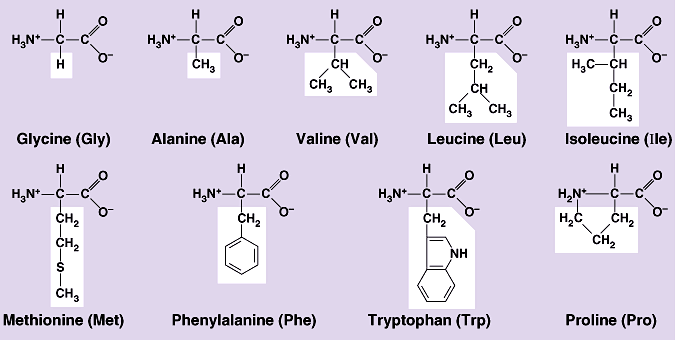
- NON-POLAR amino acids
#-># their R-groups, white on the picture below, hydrocarbon alkyl groups (alkane branches) or aromatic rings, exception: benzene ring in the amino acid Tyrosine which is polar. Aminoacids are monomers that form proteins.
Alanine, Cysteine, Glycine, Isoleucine, Leucine, Methionine, Phenylalanine, Proline, Tryptophan, Valine