The cell is the fundamental unit of life and needs energy to carry out its basic activities. This energy is provided by hydrolysis (breakdown in presence of water) of ATP.
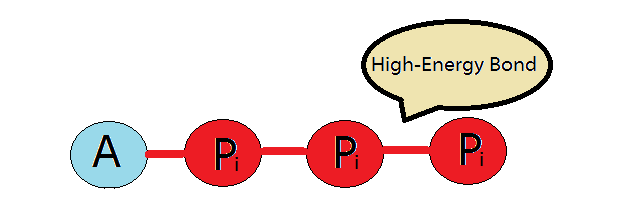
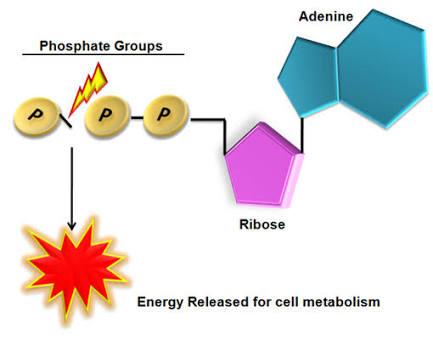
#"ATP"# is nucleotide that contains a five carbon sugar i.e ribose, an adenine nitrogen base, and three phosphate groups. These phosphate groups are attached to each other via high energy bonds. Especially the last or third chemical bond has very high amount of energy and this is the bond which is broken down.
 )
)
Now consider the rule: Bond-breaking releases energy while bond-making absorbs it.
That means, when #"ATP"# reacts with water, then the third phosphate detaches from #"ATP"# molecule, which breaks the chemical bond, and therefore a substantial amount of free energy is released. #"ATP"# being deprived of one of it's phosphate has been converted into #"ADP"# or adenosine diphosphate.
 )
)
This energy released is an abrupt source of chemical energy for cell's metabolic machinery. Some examples are: Sodium potassium#(Na^+##/##K^+)# takes place in plasma membranes of almost all cells. This pump is very salient for the cell to perform its functions. This pump requires #"ATP"# to work, Energy required for muscular contraction is also provided by this energy currency i.e #"ATP"#, It is also needed in order to synthesize polysaccharide, fats, and proteins, etc...
Hope it helps!
 )
)  )
) 

