Help! what's ionization and electronegativity?
1 Answer
Feb 23, 2018
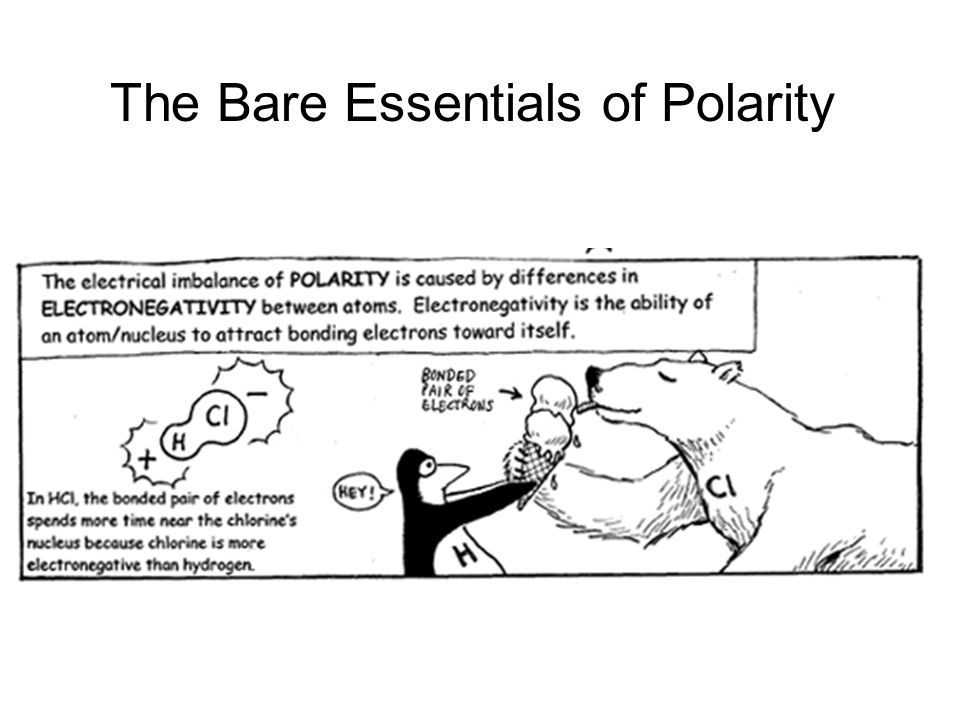
Electronegativity is an atom's ability to attract electrons when bonded with other atoms.
Ionization is the process of an atom or molecule getting a negative or positive charge by gaining or losing electrons.
Explanation:
Electronegativity in a nutshell:
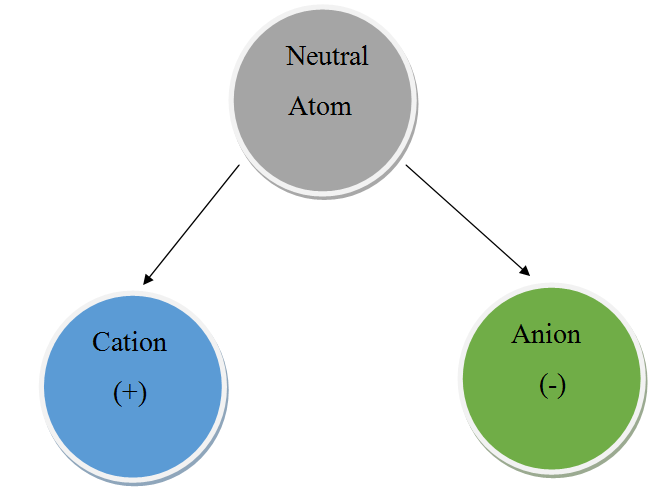
Ionization in a nutshell:
Atoms are neutral.
Ions are charged.
Ionization is when an atom (or molecule) loses an electron to become an anion or gains an electron to become a cation.