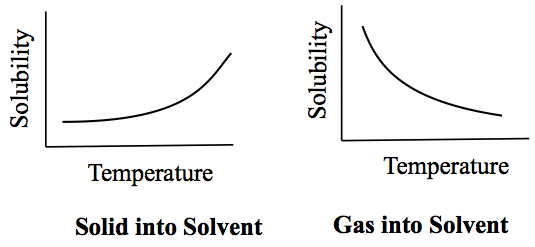
What is the relationship between the solubility and the temperature? How to explain it to a middle school student (my brother).
1 Answer
Mar 1, 2018
When temperature changes so does solubility.
Explanation:
The higher the temperature the easier a solid will dissolve.
The lower the temperature the harder it is for a solid to dissolve.
This is because the heat 'excites' the solvent making it easier for it to break apart/ split away from each other.
Meanwhile for a gas
The higher the temperature increases there is a decrease in gas solubility.
The lower the temperature the higher the gas solubility in water.