How does Methane and Diamond differ in terms of structure and properties?
1 Answer
See below :-
Explanation:
Structural Difference :-
Methane molecule
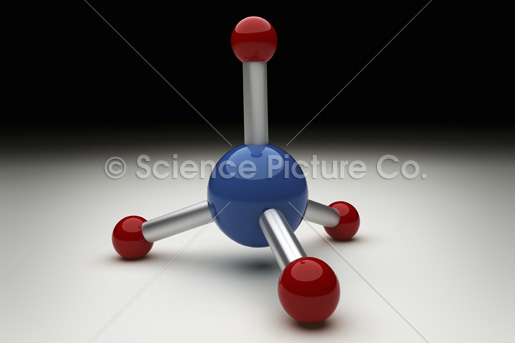
But,
Diamond does not exist as a discrete molecule. It has a

Properties :-
1) Methane is a Gas while diamond is a solid at room temperature.
2) Methane is highly flammable while diamond is not.
3) Methane reacts much easily than Diamond.
4) Methane when converted into solid form is not much hard but Diamond is the hardest substance known.

