N2o3 hybridization?
1 Answer
See below:
Warning: Somewhat long answer!
Explanation:
The first step in determining hybridization is to determine how many "charge centres" surrounds the atoms in question, by looking at the Lewis structure.
1 charge centre is the equivalent of either:
A single covalent bond.
A double covalent bond.
A triple covalent bond.
A lone electron pair.
And then Hybridization is divided into the following:
4 Charge centres:
3 Charge centres:
2 Charge centres:
Now the Lewis structure for
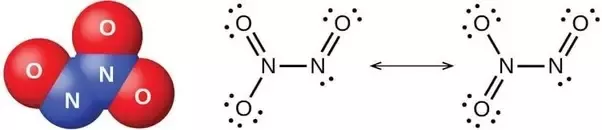
Lets begin inspecting the hybridization, starting with the leftmost Lewis structure.
One nitrogen is bonded by 1 double bond and 2 single bonds, so it must have 3 "charge centres"- it is
The other nitrogen atom is bonded by 1 single bond, 1 double bond and has a lone pair. It has 3 "charge centres" so it is therefore also
The "upper" oxygen atoms on the leftmost structure are both
However, the bottom oxygen has 3 lone pairs and 1 double bond, that makes 4- so it is
Now moving to the right structure we can see that the Nitrogen atoms are unchanged in the amount of charge centres(3), so they retain their
However, now the top-left and bottom left oxygen have a different amount of charge centres than on the left. (The rightmost oxygen is unchaged-
Now the top oxygen has 4 charge centres and is
( Note , I believe the diagram is faulty- oxygen cannot have an expanded octet so I assume that it is supposed to be
Hopefully it helped!

