How do London dispersion forces relate to the boiling point of a compound?
1 Answer
More london dispersion forces increases the boiling point.
Explanation:
A good example of this would be the difference between saturated and unsaturated fatty acids:
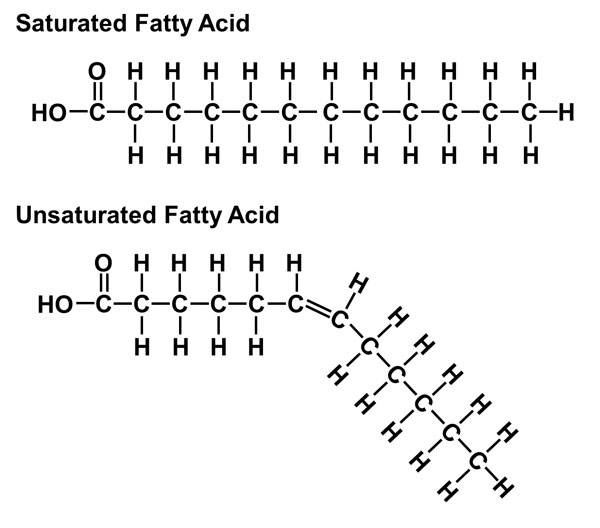
Saturated fatty acids (like those found in butter/lard) stay solid at higher temperatures than unsaturated fats (like those found in olive oil).
This is because saturated fatty acids have a more linear structure, which gives them a greater surface area. Because of this, there are more london dispersion forces between the fatty acid-molecules as they can be "packed together" more tightly due to their straight structure, yielding a higher boiling point.
Conversely, unsaturated fatty acids have a bent structure and are more "bulky". The bend gives a smaller surface area and it is also harder to "pack together" than its respecitve saturated fatty acid.- Meaning it has a lower boiling point as there is less london dispersion force between the fatty acid molecules.