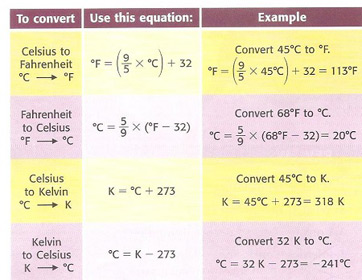
An ideal gas in a sealed container has an initial volume of 2.45 L. At a constant pressure, it is cooled to 19.00 °C where its volume is 1.75 L. What was initial temperature?
1 Answer
Aug 6, 2018
Explanation:
For a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature , assuming in a closed system.
 )
)