Question #16078
1 Answer
Explanation:
Electron affinity is the enthalpy change when 1 mol of gaseous atoms each gain an electron to form 1 mol of gaseous ions.
It is the enthalpy change for:
For fluorine the electron affinity =
For chlorine the electron affinity=
So, we can see that the value for chlorine is larger and negative compared with fluorine.
Both
But
The electron repulsions are greater in the
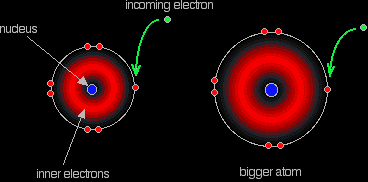
Fluorine, since it's such a small atom, has a very high electron density, which means that the repulsion the incoming electron feels will diminish the attraction coming from the nucleus and thus reduce its electron affinity.


