Question #8bd13
1 Answer
You should dilute your original solution to a total volume of
Here's how you should think about dilution calculations. Your original solution, the one you want to dilute, contains a certain amount of moles of solute - in your case, sulfuric acid.
When you dilute a solution, that certain number of moles of solute does not change, it remains constant. What changes is the concentration of the solution.
That happens because you'll have the same amount of solute in a bigger volume of solution.
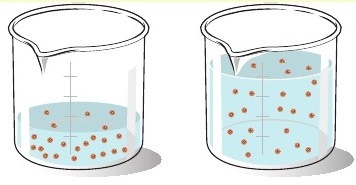
That is what the formula for dilution calculations actually expresses
Plug your data into this equation and you'll get
Rounded to one sig fig, the number of sig figs given for 30 mL, answer will be

