Is calcium oxalate soluble in water?
2 Answers
May 5, 2015
According to http://www.americanelements.com/caoxl.html , calcium oxalate,
May 5, 2015
No, calcium oxalate,
The solubility of calcium oxalate is listed at 6.7 mg/L, or
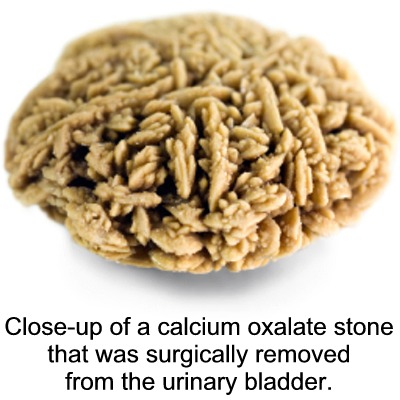
In fact, calcium oxalate is one of the major components of kidney stones.
 )
)