Question #4a21c
1 Answer
Jun 20, 2015
The mechanism of the Clemmensen reduction is not well understood.
Explanation:
The Clemmensen reduction is the conversion of an aldehyde or ketone to the corresponding by zinc amalgam and concentrated hydrochloric acid.
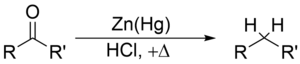
The reaction takes place at the surface of the zinc.
The mercury does not participate in the reaction. It serves only to provide a clean active metal surface.
It is thought that radicals and
One possible mechanism is shown below.


