Question #8ac35
1 Answer
Jun 18, 2015
Yes, this is an ester hydrolysis.
Explanation:
A lactone is simply a cyclic ester, so the mechanism for its hydrolysis is exactly the same as for a noncyclic ester.
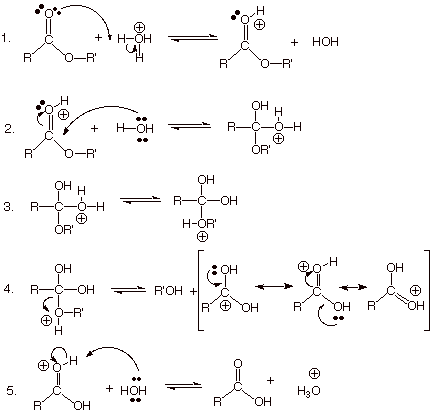
In this case, the structure of γ-butyrolactone is

The hydrolysis product is 4-hydroxybutanoic acid:
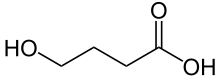
The alcohol group is acidic enough to react with
When you reduce the product, remember to add enough extra

