Question #370a7
1 Answer
Jun 26, 2015
Explanation:
By
This partly ionises in water to give ammonium and hydroxide ions:
A white precipitate of lead(II) hydroxide is formed if aqueous lead(II) ions are added:
The same reaction happens with
The sodium ions remain in solution as spectator ions.
If XS sodium hydroxide is added the precipitate redissolves to give the soluble plumbate(II) ion.
A simple way of writing this is:
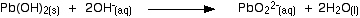
(chemguideUK)
Ammonia solution can't do this as the concentration of

