Question #e847a
1 Answer
Explanation:
Chemical formation:
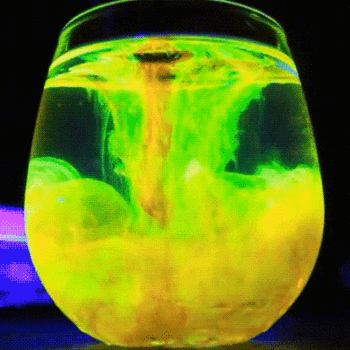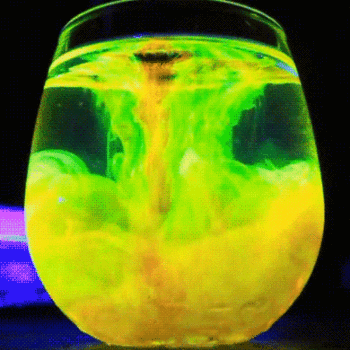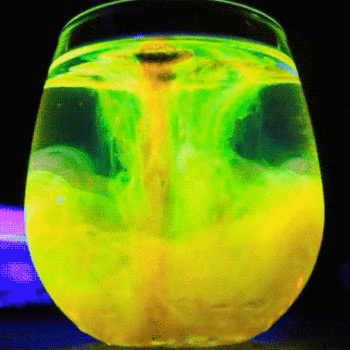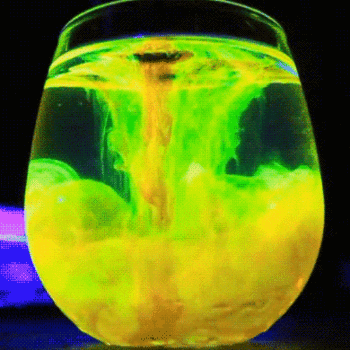
This process is described by the Schikorr reaction. Under anaerobic conditions, Iron(II) hydroxide can be oxidised by water to form iron (II,III) oxide and molecular hydrogen.
-
#3Fe(OH)_(2(aq)) -> Fe_3O_(4(aq)) + H_(2(g)) + 2H_2O_((l))# -
Iron(II) hydroxide → Iron(II,III) oxide + hydrogen + water
Reasons:
- Iron (II, III) contains iron in two oxidation states, +2 and +3.
- it contains the iron (II),
#"Fe"^(2+)# , and iron (III),#"Fe"^(3+)# , ions, which is why its chemical formula is written both as#"Fe"_3"O"_4# , and#"FeO" * "Fe"_2"O"_3# .
 )
)