Question #8862d
1 Answer
Jan 4, 2017
I predict that the product will be 2,4,6-tribromophenol.
Explanation:
The structure of sodium salicylate is

The phenol group is so activating that the ring will react at all possible ortho and para positions.
The initial product is probably sodium 3,5-dibromo-2-hydroxybenzoate.
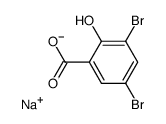
The reaction may stop at this stage but I think that even at 0 °C you also get substitution and decarboxylation of the
The arenium ion formed by attack of
The product will be 2,4,6-tribromophenol.

