Question #c1291
1 Answer
Feb 28, 2017
Here is what I get for
Explanation:
The Lewis structure of
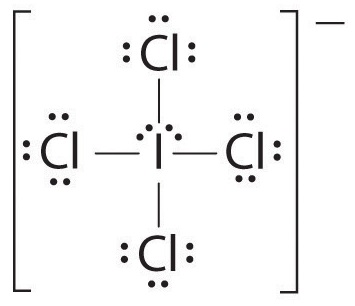
The central atom has 2 lone pairs and 4 bond pairs.
Thus, the steric number (SN) is 6, and the electron geometry is octahedral.

The lone pairs will arrange themselves at 180° to each other.
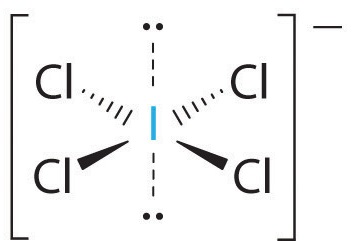
The molecular shape ignores the lone pairs, so the VSEPR shape is square planar.
The Lewis structure of
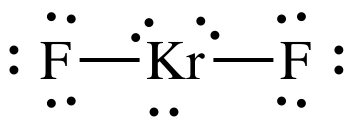
The central atom has 3 lone pairs and 2 bond pairs.
Thus, the steric number (SN) is 5, and the electron geometry is trigonal bipyramidal.

The lone pairs will arrange themselves at the equatorial positions.
(Adapted from bilbo.chm.uri.edu)
The molecular shape ignores the lone pairs, so the VSEPR shape is linear.

