What is the term symbol for #"Cr"# in #["Cr"("CN")_6]^(4-)#?
1 Answer
By recognizing that
What that means is that
Therefore, since it's a strong-field ligand and there are six
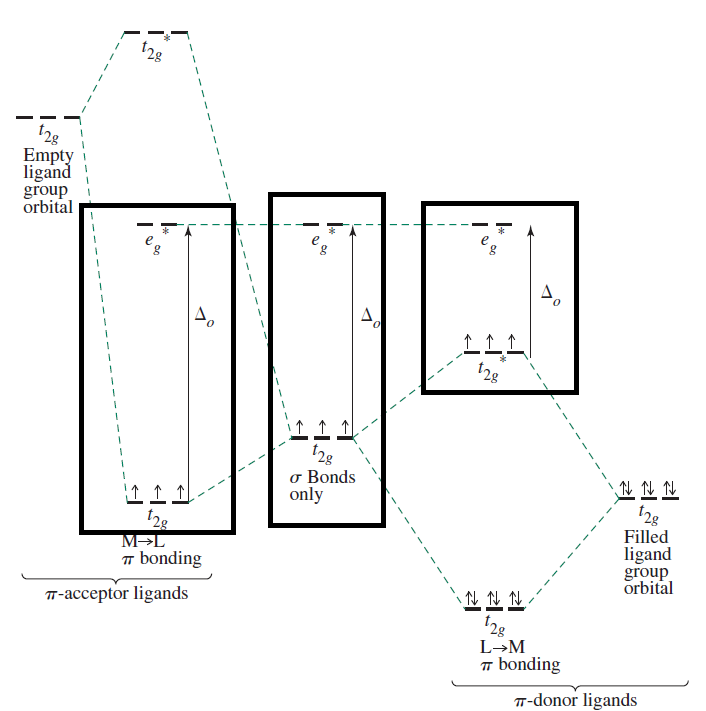
This means the electron configuration of
For low-spin, we thus have this
#ul(uarr darr)" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))#
As for the term symbol, I assume since this is a molecule, a molecular term symbol would be appropriate. But apparently, since you want the atomic term symbol, I'll give that instead...
#""^(2S+1) L_J#
#S# is the total spin angular momentum, and is calculated as#S = |M_S| = |sum_i m_(s,i)|# for electron#i# .#L# is the total orbital angular momentum, and is calculated as#L = |M_L| = |sum_i m_(l,i)|# for electron#i# .#L = 0,1,2,3,4,5, . . . # corresponds to#S,P,D,F,G,H, . . . # .#J = {|L+S|, |L+S-1|, . . . , |L-S+1|, |L-S|}# is the total angular momentum, and ranges from the magnitude of#L-S# to the magnitude of#L+S# , including integer values in between.
From the above configuration, we take the doubly-occupied orbital to have
#S = 1/2-1/2+1/2+1/2 = 1#
#2S+1 = 2(1) + 1 = 3# #=># triplet
#L = |sum_i m_(l,i)| = |-2-2-1+0| = 5# #=> H#
Thus, we do have
We don't need
#J = {5-1, 5+0, 5+1} = {4,5,6}#
That would give us, in a magnetic field, three levels, each individually triply-degenerate:
#""^(3) H_4, ""^(3) H_5, ""^(3) H_6#
From Hund's rules, we maximize
As an approximation, these orbitals have significant metal contributions (significant ionic character), so we assume they are essentially equivalent to metal
Therefore, for this determination, we approximate them as:
#underbrace(ul(uarr darr)" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(color(white)(uarr darr))" "ul(color(white)(uarr darr)))_(3d)#
As a result, it's less than half-filled, so we take the smallest
Had this been high-spin

