Question #45498
1 Answer
Here's what I get.
Explanation:
3-Ethyl-2-methylnonane
(a) Structural formula
The parent alkane is nonane.
Draw chain of 9 carbon atoms.
Number the carbons from 1 to 9 (the direction doesn't matter, although I numbered from left to right).
Put an ethyl group on carbon-3 and a methyl group on carbon-2.
(b) Condensed structural formula
You write the substituent groups in parentheses immediately after the carbon atom to which they are attached.
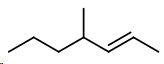
4-Methylhept-2-ene
(a) Structural formula
The hept tells you that the parent hydrocarbon contains seven carbon atoms.
Draw a 7-carbon chain.
Number the carbon atoms from 1 to 7.
The 2-ene tells you that there is a
Now, attach a methyl group to carbon 4.
![4Me]
( )
)
(b) Condensed structural formula
You can omit single bonds, but you can't omit double bonds.
The condensed structural formula is
5,6-Dimethyl-2-octane
There is no compound with this name.
1,3-Diethylhexane
This is an incorrect name.
Start with a 6-carbon chain.
Add ethyl groups at carbons 1 and 3.
We see that the longest continuous chain consists of 8 carbon atoms.
The correct name is 4-ethyloctane.
(b) Condensed structural formula

