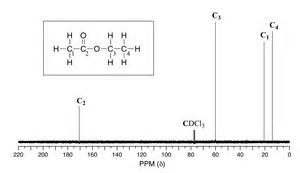
For #"H"_3"CCH"_2"O(O=)CCH"_3# there should be 4 singlets in the #""^13"C"{""^1"H"}# #"NMR"# spectrum, and four only. It might be hard to see the carbonyl carbon, but this will occur well downfield (and in the spectrum pictured, the carbonyl carbon is quite distinguishable; of course with reagents like this, which are available in quantity, you make a solution of almost 100% sample with a drop or 2 of lock solvent).
In the #"DEPT-135 sequence"#, the #"methylene carbon"# of the ethyl residue should be negative.
 )
)
Why does the #d_1-"chloroform"# signal appear as a triplet?
 )
) 
