Question #7a492
1 Answer
You use fractional distillation for separating homogeneous mixtures of liquids.
Explanation:
Ideally, the difference in boiling points of the components should be more than 25 °C.
However, if you have an efficient fractionating column and work slowly, you can get good separation of liquids with closer boiling points.
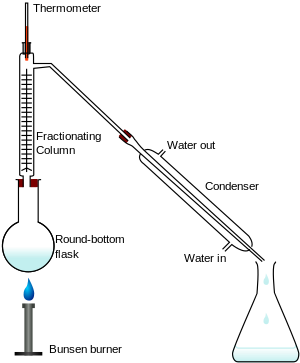
Fractional distillation involves the use of a fractionating column that is packed with glass beads, steel mesh, or other substances to provide a greater surface area on which the rising vapours can condense.
The vapours of the higher-boiling compound will condense and run back into the distillation flask, while those of the lower-boiling compound will make it to the distillation head and continue on to the condenser.
Once the lower-boiling component has distilled off, the temperature will rise and the higher-boiling component will distill over.

