What would happen if #"HCl"# is added to a solution of cobalt (II) ions?
1 Answer
The green complex
Explanation:
I am assuming we are in aqueous conditions.
The aqueous copper(II) ion consists of a central
It has the formula

If a large excess of chloride ions is added the water ligands are displaced as the following equilibrium is established:
Concentrated hydrochloric acid contains a large amount of chloride ions so Le Chatelier's Principle tells us that adding this will cause the position of equilibrium to shift to the right producing green
They have a tetrahedral structure:
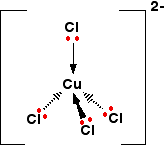
The solution looks like this:

If excess water is now added the position of equilibrium is driven back to the left and the blue colour returns.

