Question #4b778
1 Answer
Explanation:
You know that your atom contains
Now, the atomic number of an element,
#"atomic number" = Z = "no. of protons"#
In your case, you know that the atom contains
#Z = color(red)(4)#
The mass number of an atom,
#"mass number" = A = "no. of protons" + "no. of neutrons"#
Since you already know that
#Z = "no. of protons"#
you can say that you have
#A = Z + "no. of neutrons"# 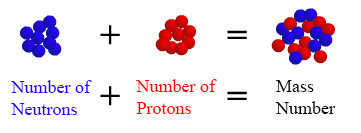
This tells you that if you add the number of neutrons present inside the nucleus and the atomic number of the element, you get the mass number of the atom.
In your case, you will have
#A = color(red)(4)color(white)(.)"protons" + color(blue)(5)color(white)(.)"neutrons"#
#A = 9#
Therefore, your atom will have an atomic number equal to

