Question #265c0
1 Answer
Here's how you can do that.
Explanation:
Your starting point here will be the ideal gas law equation, which looks like this
#color(blue)(ul(color(black)(PV = nRT)))#
Here
#P# is the pressure of the gas#V# is the volume it occupies#n# is the number of moles of gas present in the sample#R# is the universal gas constant, equal to#0.0821("atm L")/("mol K")# #T# is the absolute temperature of the gas
Now, let's say that the given mass of gas is
#n = m /M_M#
Plug this into the ideal gas law equation to get
#PV = m/M_M * RT#
Next, divide both sides of the equation by
#(PV)/T = m/M_M * R#
The molar mass of the gas, which tells you the mass of exactly
#(PV)/T = overbrace(R/M_M)^(color(blue)("constant")) * m#
#(PV)/T = color(blue)("constant") * m#
This means that for a given mass
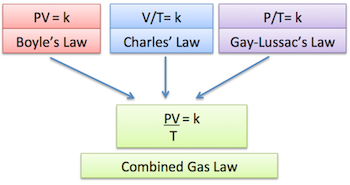
#(PV)/T = color(blue)("constant")#
This is the combined gas law equation and it tells you that for a given mass of gas
#color(blue)(ul(color(black)((P_1V_1)/T_1 = (P_2V_2)/T_2)))#
Here
#P_1# ,#V_1# ,#T_1# are the pressure, volume, and absolute temperature of the gas at an initial state#P_2# ,#V_2# ,#T_2# are the pressure, volume, and absolute temperature of the gas at a final state