Question #ec927
1 Answer
Their distance in the periodic table...and...HCl (most bond polarity).
Explanation:
 )
)
This table shows that electronegativity increases diagonally...
 )
)
Or more appropriately this one...
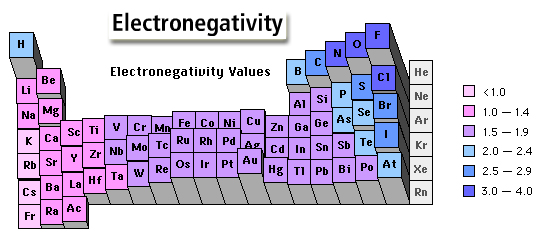
Now, just imagine the Hydrogen to be somewhere near Boron, you can calculate the bond polarity.
Example Question (with real values on Pauling Scale) : -
#N_2# (0)#-># Now way! They are the same atoms...#BH_3# (0.2)#-># Not Much... ( remember, I told you to imagine the Hydrogen to be somewhere near Boron )#HCl# (1.0)#-># Yeah... Quite Much...#CH_4# (0.4)#-># Bit more than#BH_3# ....


