A 16.0 L gas cylinder is filled with 7.60 moles of gas. The tank is stored at #27^oC#. What is the pressure in the tank?
1 Answer
Explanation:
Because we are given the volume, number of moles, and temperature, we will have to use the ideal gas law equation:
 )
)
Based on the units that are associated with each variable, the volume and number of moles have good units. The temperature has to be converted into Kelvins; we can do this by adding
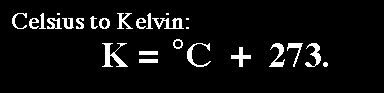
Now we know V,T, n, and R.
R has the same value no matter what chemical species you are dealing with.
All we have to do is rearrange the equation to solve for P. We can do this by dividing by the volume on both sides of the equation:
Finally, plug in your known values like so:

