A 425 mL volume of hydrogen chloride gas, #HCl#, is collected at 25°C and 720 torr. What volume will it occupy at STP?
1 Answer
The volume at STP will be 370 mL, rounded to two significant figures.
Explanation:
Since your question involves the pressure, volume, and temperature of HCl gas, you will need to use the combined gas law in order to solve this problem. Temperature will need to be converted to Kelvins and pressure will need to be converted to kPa.
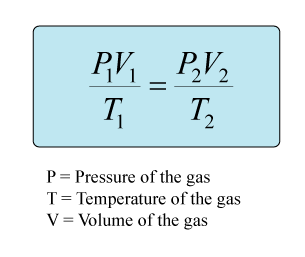
The current value for STP as determined by the IUPAC (International union of Pure and Applied Chemistry), is
Known
Unknown
Rearrange the equation in order to isolate

