A mixture of two gases has a total pressure of 6.7 atm. If one gas has a partial pressure of 4.1 atm, what is the partial pressure of the other gas?
1 Answer
Jul 14, 2016
The partial pressure of the other gas is
Explanation:
Before we begin let me introduce Dalton's Law of Partial Pressures equation:
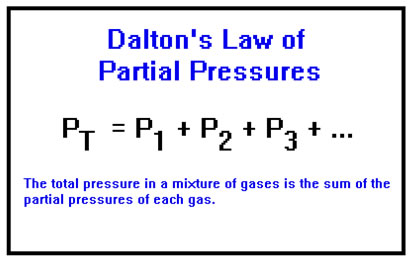
Where
Based on what you've given me, we know the total pressure,
Therefore,

