A #sigma# bond is the same thing as a single bond. So what is a #pi# bond?
1 Answer
Here's the answer...
Explanation:
#color(red)(sigma) "bonds"# are the formed by the overlapping of the#(s-p)# or,#(s-s)# orbitals by head on overlapping...such as in the picture...
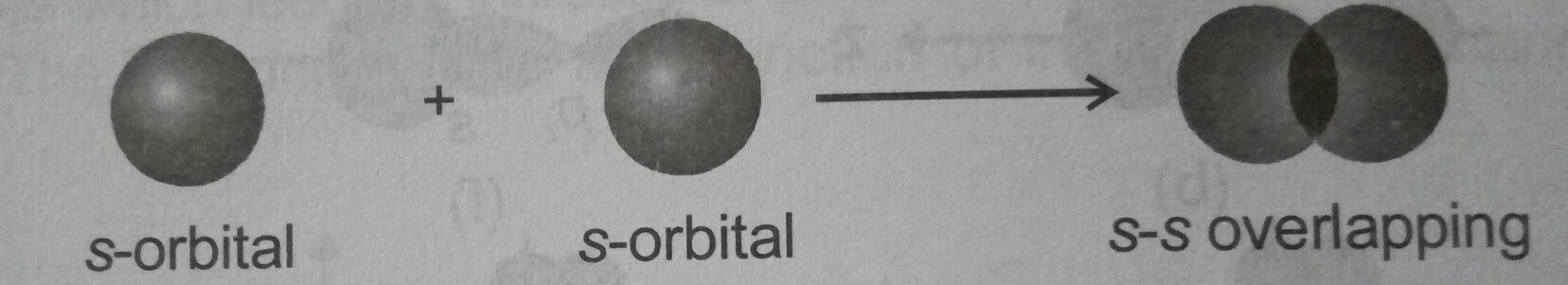
#" "# (S-S) OVERLAPPING
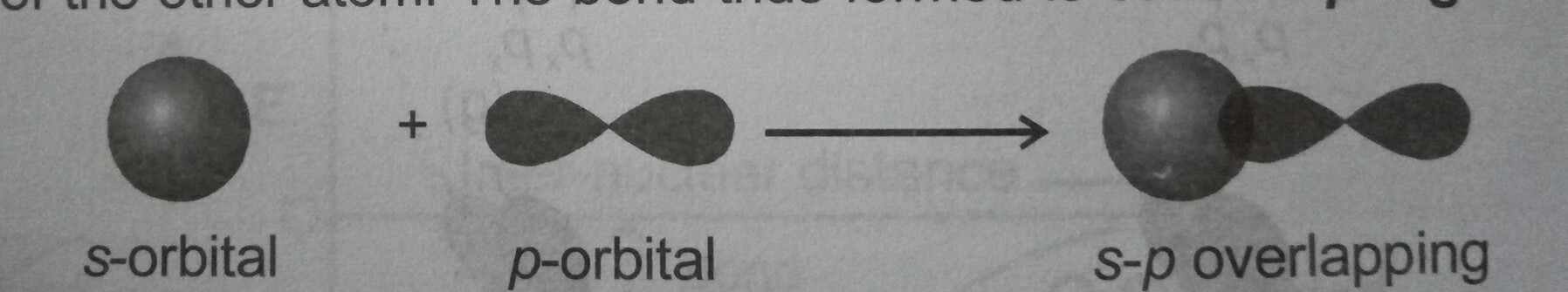
#" "# (S-P) OVERLAPPING

#" "# (P-P) OVERLAPPINGThese three types of structure are mainly involved in producing
#color(red)(sigma)"bonds"# ....which emans single bonds...But some time we see that there is
#C-=C # bonds or,#C=C# bonds...
#" "# Here in the first case ,there is one#sigma# bond but the other bond is the# pi# bond who contains loose electrons but strengthens the whole bond formation...
#" "# In the#2nd# case there is two#pi # bonds... which produces by following type of overlapping(#color(red)("(side-wise overlapping)"# ...
Hope it helps...
Thank you...

