All alkali and alkaline earth metals form ionic hydrides except Be and Mg WHY?? Please reasons, thanks
1 Answer
Two reasons: (a) ionization energies and (b) electronegativities.
Explanation:
(a) Ionization energies
Of all the Group 1 and Group 2 metals,
Thus, it is more difficult to convert them into cations.
(b) Electronegativities
Of all the Group 1 and Group 2 metals,
Thus, their electronegativities are the closest to that of
They are therefore the most likely candidates to have covalent
Calculations from electronegativity differences predict a
only 9 % ionic character and an
The structure of

Beryllium hydride forms polymeric chains in which the
form 3-centred 2-electron bonds to the next beryllium atom in the chain.
Here's a model of a chain segment.
(From www.chemtube3d.com)
The structure of
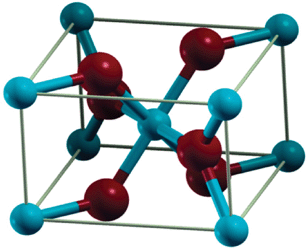
The
However, the
Instead there is a significant amount of charge density between

