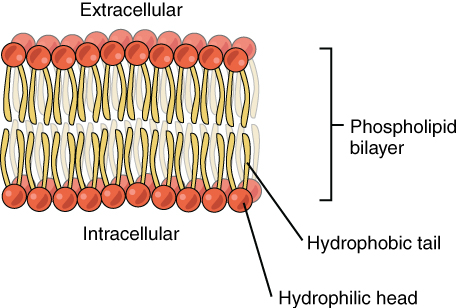
Are the H2O molecules are too large to pass between the phospholipids while the molecules of CO2 and O2 are not?
1 Answer
Feb 24, 2018
I wouldn't call a water molecule large in relation to
I would call them polar, though,
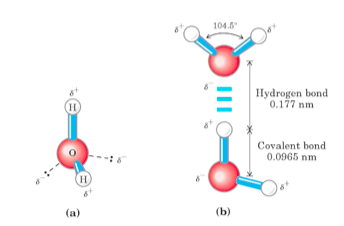
The polarity of these molecules makes it very hard to pass through the largely hydrophobic interior of the phospholipid bilayer,