Can I determine the ion size by charge?
1 Answer
You cannot predict ion size as a function of charge, but you can make statements about some general trends.
In general, ionic radius decreases with increasing positive charge. As the charge on the ion becomes more positive, there are fewer electrons. The ion has a smaller radius.
You can see this trend in the transition metals. For example, the radii (in picometres) that correspond to various charges are:
Ti: 0 = 176; 2+ = 100; 3+ = 81; 4+ = 74
V: 0 = 171; 2+ = 93; 3+ = 78; 4+ = 72; 5+ = 68
Cr: 0 = 166; 2+ = 87; 3+ = 76; 4+ = 69; 5+ = 63; 6+ = 58
In general, ionic radius increases with increasing negative charge. As the charge on the ion becomes more negative, there are more electrons. The ion has a larger radius.
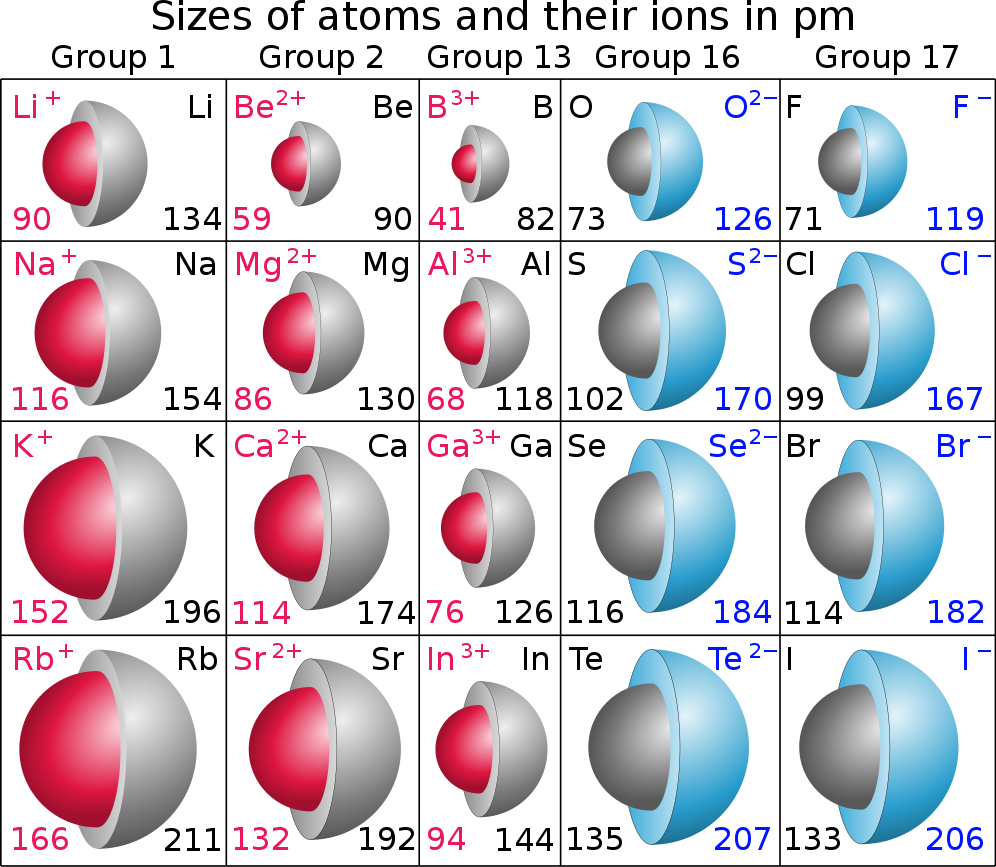
Cations are smaller than the neutral atoms. Anions are larger than the neutral atoms.

