Can the ideal gas law be applied to liquids?
1 Answer
The Ideal Gas Law cannot be applied to liquids.
The Ideal Gas Law is
That implies that
The Ideal Gas Law doesn't even apply to "real" gases like hydrogen, oxygen, and nitrogen. At ordinary pressures and temperatures, real gases are almost ideal, but they don't obey the Ideal Gas Law perfectly. Real gases always deviate from ideal behaviour.
One way to test the Ideal Gas Law is to rearrange it to get
and measure the value of
Instead, we get the following set of graphs.
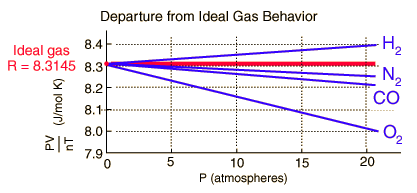
It all depends on the percentage deviation you are willing to accept before you call the gas non-ideal. Your instructor might accept a deviation of 5 %. A research chemist might not accept a deviation of 0.05 %.

