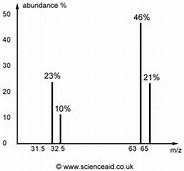
Copper has an atomic mass of 63.55 amu and two naturally occurring isotopes with masses 62.94 amu and 64.93 amu. Which mass spectrum is most likely to correspond to a natural occurring sample of copper?
1 Answer
May 28, 2017
You would observe metal peaks at
Explanation:
And the relative proportion of the two peaks would reflect the natural isotopic abundance.