Did Robert Millikan's postulates form the most accepted theory for atomic structure?
1 Answer
Jun 25, 2017
From what I know, Millikan's big discovery was the mass of an electron. Rutherford proposed the atomic structure theory from his famous gold foil experiment, see below.
Explanation:
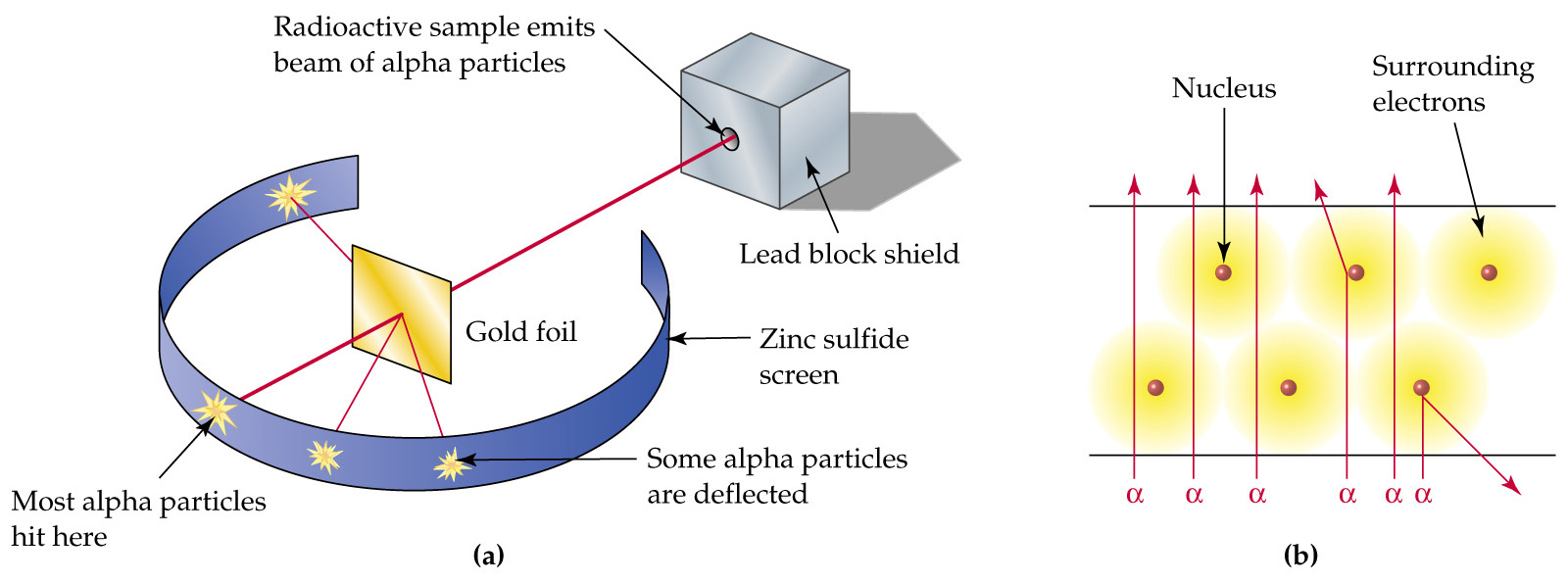
Alpha particles are emitted toward a piece of gold foil, some deflect, as seen in the figure, but most go through. Thomson's model predicted none would deflect, so Rutherford explained this with a much smaller, positively charged nucleus that deflected the positively charged alpha particles.
A better view of his model is in (b); the cloud is a mere probability of where the electrons are, and the nucleus is exponentially smaller than that cloud.

